What makes hydrogen fuels “clean”? How can they help reduce emissions? Here are the answers.
While energy is a vital component for keeping human activities going, the sources consumed for its production continue to be oil and coal — both of which are carbon-intensive.
As the climate movement continues to strengthen, the calls for sustainable solutions in the energy industry have also intensified. With this, the interest for renewable energy sources and electric vehicles has grown in recent years.
Another alternative which has been gaining attention is the use of hydrogen and fuel cells. In their infographic, the Canadian Hydrogen and Fuel Cell Association (CHFCA), summarises how this can catalyse a clean energy transition.
The chemistry of hydrogen fuels
Apart from the benefits mentioned by CHFCA, you might also be curious how hydrogen is considered a clean fuel.
From our partners:
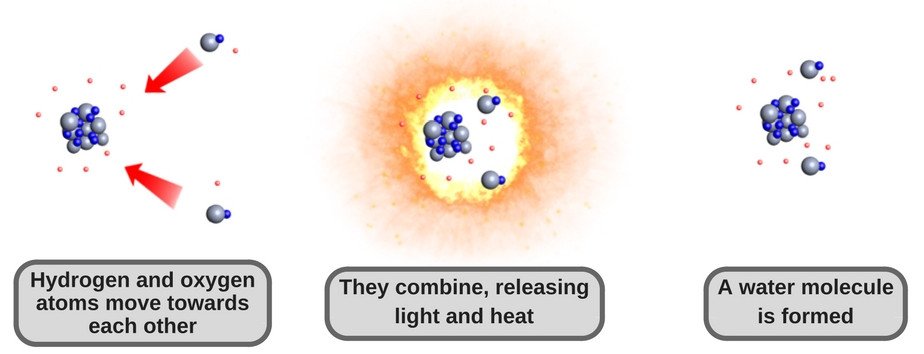
Source: Science ABC
When hydrogen is burned, it combines with oxygen — which is true for all combustion processes. This chemical reaction (as illustrated above) releases energy in the form of light and heat. This chemical reaction is also the central process in fuel cells.
Aside from energy, the sole product of the reaction is water. With no carbon involved in the chemical reaction, there will be no harmful emissions to worry about.
Not completely carbon-free
This is not to say that there are no emissions involved in producing hydrogen fuels. Right now, there are two main methods used: natural gas reforming and electrolysis.
In natural gas reforming, hydrogen is derived from methane, a carbon-containing compound. The series of chemical reactions used for the extraction has hydrogen and carbon dioxide as products. While this may be the case, the total emissions are still cut in half compared to traditional methods.
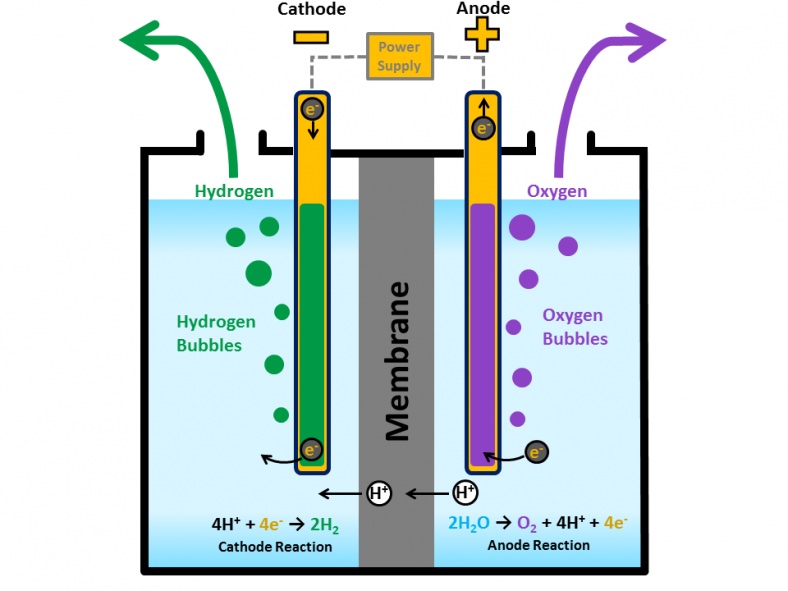
Meanwhile, in the production of hydrogen through electrolysis, electricity is used to break down water into its two components: hydrogen and oxygen.
The reaction used for this route can be thought of as the reverse process involved in the combustion of hydrogen we have shown earlier. This means that there are no greenhouse gas emissions involved in the reaction.
Yet, since electricity is involved in the reaction and given how today electricity is still mainly produced through oil and coal, emissions are still present if the entire process is considered.
Workaround
Knowing this, it seems like a zero-carbon economy is out of reach. Fortunately, there is a workaround. The road to zero carbon emission may be found in the synergy of hydrogen fuels and renewable energy.
If our power grids undergo a renewable energy transition, there is a potential to overcome the drawback of producing hydrogen through the electrolysis pathway. On the other hand, hydrogen fuels can help address the limitations of renewable energy sources like wind and solar power in a sustainable manner.
Seeing all of these benefits and existing solutions, it is clear that hydrogen has the potential to usher an era of clean energy. However, this future does not come easy. It is only possible through intensified research, expansion of large-scale infrastructure, increased funding, and strong political will.













